Trad KS, Fox MA, Simoni G, Shughoury AB, Mavrelis PG, Raza R, Heise JA, Barnes WE
Surgical Innovation (2018) https://doi.org/10.1177/1553350618755214 [epub ahead of print]
 Background.
Background.
Questions remain about the therapeutic durability of transoral incisionless fundoplication (TIF). In this study, clinical outcomes were evaluated at 5 years post-TIF 2.0.
Methods.
A total of 63 chronic gastroesophageal reflux disease (GERD) sufferers with troublesome symptoms refractory to proton pump inhibitor (PPI) therapy, absent or ≤2 cm hiatal hernia, and abnormal esophageal acid exposure were randomized to the TIF group or PPI group. Following the 6-month evaluation, all patients in the PPI group elected for crossover to TIF; therefore, all 63 patients underwent TIF 2.0 with EsophyX2 device. Primary outcome was elimination of daily troublesome regurgitation and atypical symptoms at the 5-year follow-up. Secondary outcomes were improvement in symptom scores, PPI use, reoperations, and patient health satisfaction. The cost-effectiveness of TIF 2.0 was also estimated.
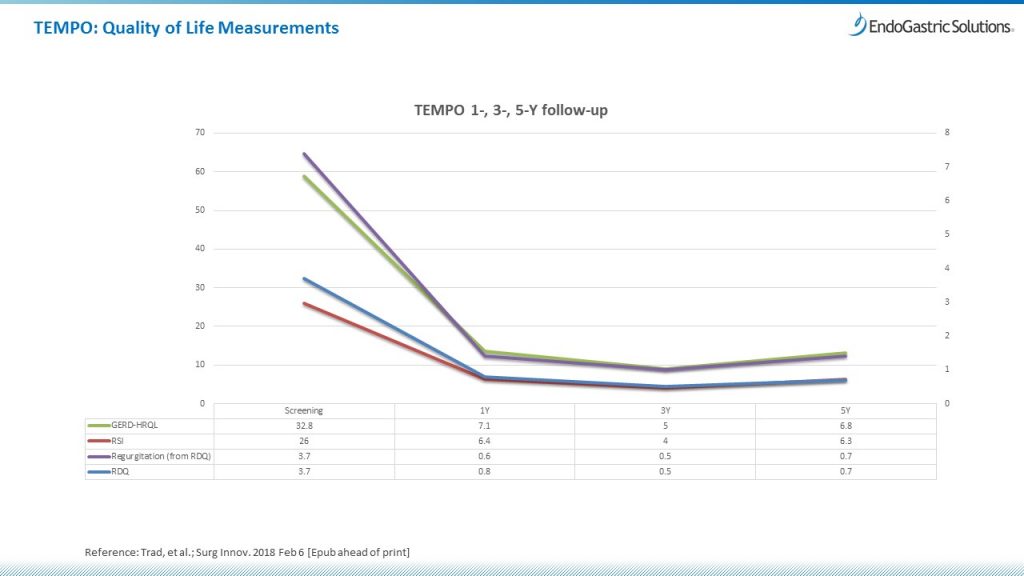
Results.
Of 63 patients, 60 were available at 1 year, 52 at 3 years, and 44 at 5 years for evaluation. Troublesome regurgitation was eliminated in 88% of patients at 1 year, 90% at 3 years, and 86% at 5 years. Resolution of troublesome atypical symptoms was achieved in 82% of patients at 1 year, 88% at 3 years, and 80% at 5 years. No serious adverse events occurred. There were 3 reoperations by the end of the 5-year follow-up. At the 5-year follow-up, 34% of patients were on daily PPI therapy as compared with 100% of patients at screening. The total GERD Health-related quality-of-life score improved by decreasing from 22.2 to 6.8 at 5 years (P < .001).
Conclusion.
In this patient population, the TIF 2.0 procedure provided safe and sustained long-term elimination of troublesome GERD symptoms.
Link to open access article: Trad KS, et al. Surg Innov 2018 Feb 6. [Epub ahead of print]